Background and Motivations
At the Regenstrief Institute, Inc., we imagine a world where health is optimized for all through a seamless network of healthcare information systems. Optimal health requires an ecosystem of electronic interoperability: where data such as labs, vitals signs, and clinical documents, can be exchanged and understood by systems made by many vendors. But data producers have different ways of identifying the same test or measurement. We have effectively created isolated data islands, which limits the potential of clinicians, consumers, and caregivers to improve and manage health.
A rich trove of standardized variables
LOINC is a large catalog of standardized variables for clinical tests and observations, from lab to lifestyle and genetics to environment.
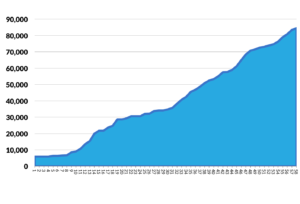
In June 2017, Regenstrief will publish the 59th release of LOINC containing over 84,00 variables.
Laboratory content (observations on a specimen) includes chemistry, microbiology, drug/tox, hematology, molecular pathology, etc.
Clinical content (observations about people, populations, and the environment) includes vital signs, clinical measures, radiology procedures, document titles, patient-reported outcomes, etc.
LOINC contains extensive metadata about each term, including display names, example units of measure, structured answer lists, synonyms, and more. LOINC has codes for discrete observations plus collections of terms like laboratory panels and patient assessment instruments.[PMID: 22899966]There are now 11,000+ carefully crafted term descriptions like the example below:
[84921-6] Cells.chromosome region 7q31 deletion/Cells counted in Blood or Tissue by Fluorescent in situ hybridization (FISH)
The percent of cells in blood or tissue containing the 7q31 deletion can be detected by FISH and used for the diagnosis of acute myeloid leukemia or myelodysplastic syndrome. This term is used to report the number of cells that have the 7q31 deletion out of total number of cells examined (e.g. 80 out of 100 cells, or 80%). Results are also typically reported in ISCN (International System for Human Cytogenetic Nomenclature) format [LOINC: 62356-1]. This term is based, but not limited in use to, the Vysis LSI D7S486/CEP 7 probe kit. To report percentage results for the CEP7 probe, used [LOINC: 84922-4].
LOINC grows because users ask
LOINC uses a community-driven development approach, and users continue to request new content in many domains.
The diagnostic testing industry continues to innovate and new lab tests are always emerging. Many requests now come directly from test manufacturers. Public health continue requesting codes for emergent issues, surveillance, and case reports. Genetic tests for detecting inherited human disorders, somatic mutations (cancer), and the presence of infectious organisms have burgeoned. In the last decade, we increased by four fold the number of molecular pathology terms in LOINC, and have been active in Health Level 7 (HL7)’s work to define new data models for reporting genetic test results. Other growing areas include social determinants of health, patient-reported outcomes, clinical document titles, and structured pathology reporting for cancer.
LOINC is a growing global community
LOINC is used in diverse settings: reference labs, radiology centers, federal agencies, health care organizations, professional societies, data exchange networks, insurance companies, health IT vendors, in vitro diagnostic vendors, and more.
More than 6,000 new users register each year.
Use in the United States
Federal regulations in the EHR “Meaningful Use” program have required LOINC in electronic quality measures, messages reporting lab tests, exchanging medical summaries, and sending data to cancer registries and public health agencies. The Food and Drug Administration uses LOINC in structured product labeling, and will require it for lab data in clinical trials effective March 2018. The Centers for Disease Control and Prevention uses LOINC in electronic laboratory reporting, case reporting, and other national systems. The ONC’s 2017 Interoperability Standards Advisory lists LOINC for many interoperability needs, including functional status, laboratory tests, imaging diagnostics, nursing observations, vital signs, and social determinants of health. The Centers for Medicaid and Medicare Services adopted LOINC for the patient assessment instruments required in post acute care settings. Large scale research networks such as PCORnet, OHDSI, and the FDA’s Mini-Sentinel all use LOINC. LOINC has been endorsed by the College of American Pathologists, the American Nurses Association, American Academy of Nursing, and other professional bodies.
*See Appendix A for additional detail on LOINC’s U.S. federal collaborations.
International use
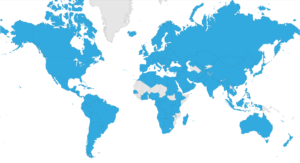
LOINC is also flourishing internationally. Fueled by an innovative process, there are translations into 18 variants of 12 languages.[PMID: 22285984] It is an official national standard in more than 30 countries. A recent Organisation for Economic Cooperation and Development study highlighted it as the international standard for lab results. LOINC is used in national patient record programs (e.g. Australia, Austria, Italy), maternal health programs (e.g. Rwanda), surveillance networks for antibiotic resistance (e.g. Netherlands), national insurance programs (e.g. Saudi Arabia), patient summaries for cross-border care, reporting lab results (e.g. Turkey, Qatar), and more.
We’re committed to working together with other standards developers
Health Level Seven (HL7)
Regenstrief and HL7 have a long-standing relationship to develop complementary health data standards. A few joint work highlights include: clinical genomics guides, claims attachments specifications, and approaches for representing vocabulary standards in FHIR terminology services.
SNOMED International
In 2013, Regenstrief and SNOMED International (formerly IHTSDO) formed a landmark long-term collaborative relationship to link the rich clinical semantics of SNOMED CT to LOINC, which provide extensive coverage of laboratory tests and clinical measurements.
IEEE Standards Association
Regenstrief Institute, developers of LOINC, and the IEEE Standards Association, developers of the 11073™standards, have signed a memorandum of understanding to enhance the interoperability of traditional medical devices and personal health devices.
IVD Industry Connectivity Consortium (IICC)
Regenstrief worked with the IICC on a new specification for publishing vendor IVD tests associated with a set of LOINC codes that identify the distinct observations produced by the test. This standard will greatly improve the efficiency and consistency with which laboratories can deploy LOINC.
Radiological Society of North America (RSNA)
Regenstrief and the RSNA are unifying the RadLex™Playbook and LOINC radiology terms to produce a single, comprehensive standard for radiology procedures with a shared governance.
Health Standards Collaborative (HSC)
Regenstrief is an active member of the HSC, which provides an executive forum for senior leadership of the U.S. healthcare standards development community to improve interoperability.
Many tools for LOINC implementers
Regenstrief develops and makes available at no cost a large toolbox of resources for implementers.
Regenstrief LOINC Mapping Assistant (RELMA)
RELMA is the “gold standard” desktop mapping program for linking local test codes to LOINC.
Online Search App (search.loinc.org)
Search the latest version of LOINC from your browser – fast. No software install necessary.
Community and Educational Resources
The LOINC website is loaded with resources, including a tutorials, videos, user forum, and more.
Other LOINC-related software
Regenstrief also hosts a FHIR vocabulary server of LOINC subsets, open source data capture widget software developed by NLM called LHC-Forms, and a community mapping repository.
Key funding support from HHS and others
As a non-profit, Regenstrief is able to develop and make LOINC available at no cost because of key financial support from many, including the National Library of Medicine, Regenstrief Foundation, NIDDK, NIBIB, NCCIH, ASPE/CTSA, CMS, bioMérieux, and the LOINC community.
The road ahead
Undoubtedly, LOINC is improving healthcare within the U.S. and around the world by making health data more fluid and understandable to different computer systems. Regenstrief remains committed to developing LOINC openly and making it available at no cost. As new users and new content requests continue to grow, standards like LOINC are at risk for suffering from the tragedy of the commons. Yet, such standards are essential infrastructure for the health ecosystem and necessary ingredients for true and meaningful interoperability. Federal support and adoption of clinical vocabulary standards remains vital for progress towards the vision of optimal health.
Appendix A. Many successful collaborations with federal partners
Regenstrief has a long history of collaborating with and supporting the efforts of federal agencies. We have worked extensively with the VA and DoD about LOINC laboratory, radiology, and clinical document codes. We worked with the Indian Health Service as they mapped all of the laboratory data from its 250 facilities to LOINC. The CDC has many programs that are implementing LOINC. We have worked with many U.S. federal agencies and NIH institutes that are using LOINC to catalog their common data elements.
A brief summary is included below.
| Institute/Agency | Project(s) using LOINC | Comments |
| National Library of Medicine | Unified Medical Language System (UMLS) | NLM has funded LOINC development for many years. LOINC has long been a source vocabulary for the UMLS. MedlinePlus Connect helps patients and health care providers access consumer health information, and identifies lab tests with LOINC. The NIH Common Data Elements (CDE) Repository provides access to structured human and machine-readable definitions of data elements, many based on their representation in LOINC. |
| NIH Common Fund | Patient-Reported Outcomes Measurement Information System (PROMIS) | All of the PROMIS questions, answers, and forms/item banks were added to LOINC with support from an ARRA CTSA Supplement award. |
| NIH Office of the Director, with National Center for Biotechnology Information (NCBI) | Genetic Testing Registry (GTR) | Lab tests used to diagnose genetic conditions are being linked to LOINC codes. There are also links back to the loinc.org reference page for that test. For example: GLA (Alpha-Galactosidase A) Gene Testing in Fabry Disease |
| National Human Genome Research Institute (NHGRI) | PhenX (consensus measures for Phenotypes and eXposures) | All of the PhenX questions, answers, and collections were added to LOINC with support from an ARRA CTSA Supplement award. |
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | National Kidney Disease Education Program (NKDEP)’s Chronic Kidney Disease Care Plan | Regenstrief is working with the NIDDK to develop a CKD care plan template, including identifying and developing LOINC codes for relevant data elements. |
| National Institute of Neurological Disorders and Stroke (NINDS) | Quality of Life in Neurologic Disorders (NeuroQOL) | All of the NeuroQOL questions, answers, and collections were added to LOINC with support from an ARRA CTSA Supplement award. |
| National Cancer Institute (NCI) | cancer Biomedical Informatics Grid®(caBIG) | A 2009 paper published in J Biomed Inform describes the process by which LOINC was selected into the caBIG architecture PMID: 19154797. |
| Eunice Kennedy Shriver National Institute of Child Health and Human Development | Hemaglobinopathies | The work on hemaglobinopathies was done with the American Health Information Community (AHIC) Personalized Health Care Work Group and was included in the development of standards for electronic information exchange of newborn screening test results. |
| Health Resources and Services Administration (HRSA), with National Library of Medicine | Newborn screening | LOINC identifies the lab tests in the Newborn Screening Coding and Terminology Guide. These standards can speed the delivery of newborn screening reports and facilitate the care of infants with positive test results. |
| National Center for Advancing Translational Sciences (NCATS) | Many CTSA institutions use LOINC in their i2b2 systems
Funded project to develop LOINC “equivalence classes”. |
Many institutions in the CTSA program use LOINC in their i2b2/SHRINE systems, and LOINC is supported as a core ontology. As a supplement to the Indiana CTSA award, we are creating a roll-up mechanism for LOINC codes considered equivalent for specific purposes. |
| National Eye Institute (NEI) | EyeGENE data elements | We have begun a collaboration to represent all the eyeGENE data elements in LOINC. |
| Centers for Disease Control and Prevention (CDC) | All of their Public Health Information Network initiatives use LOINC, including reportable diseases, case reports, immunization messaging, National EMS Information System, etc. | CDC has been a long-standing adopter and promoter of LOINC in many contexts. |
| Centers for Medicare and Medicaid Services (CMS) | EHR Meaningful use program, LOINC has represented all of their required assessment instruments (e.g. MDS, OASIS, IFR-PAI, CARE) | The EHR Meaningful Use incentive program is a major driver of Health IT adoption in the US. We have also been funded to model their assessment instruments in LOINC through collaboration with ASPE, AHIMA, and RTI. |
| Food and Drug Administration (FDA) | Structured Product Labeling (SPL)
Laboratory test results |
Headings in SPL, the mechanism for exchanging product information, use LOINC codes. LOINC is a recognized and recommended FDA standard for lab tests, and will be required in clinical trial submissions beginning in 2018. |
| Substance Abuse & Mental Health Services Administration (SAMHSA), with the Society for Behavioral Medicine | Common data elements for health behaviors and psychosocial factors | In collaboration with this effort we created LOINC codes for their entire set of common data elements. The LOINC codes can be viewed here. A background publication about the data elements is PMID: 22511015. |
| Department of Defense (DOD) | Data exchange for lab results, radiology reports, and document titles. | An early paper describing their mapping of lab terms to LOINC is PMID: 16779076. A March 2012 newsletter highlights LOINC use by the DOD. |
| Veterans Administration (VA) | Data exchange for lab results, radiology reports, and document titles. | Read about VA’s use of LOINC from their entry in the LOINC Adopter Directory. They have been a major contributor to the work in LOINC to develop an ontology of document titles for clinical notes. |
| Office of the National Coordinator for Health IT | Meaningful Use Standards and Certification Criteria Interoperability Standards Advisory |
The Standards and Certification Criteria require use of LOINC for many purposes. Across many clinical domains, the ONC’s ISA lists LOINC as the vocabulary standard for observations and measurements. |