In a November 6th letter to the Office of the National Coordinator for Health Information Technology (ONC), Regenstrief provided comments on the Draft 2016 Interoperability Standards Advisory (2016 Advisory). I believe that the goals of the Advisory are laudable: to provide a single public list of best available standards and to promote dialogue.
The 2016 Advisory is ONC’s second iteration, and overall it is better. This version includes some important structural changes that make the content more useful and each standard’s current state more transparent. I think it can be improved further, and here I’ll summarize the key points.
A welcome addition
For each recommended standard, ONC has added a set of six informative characteristics to give readers an overall sense of maturity and adoptability. They have also added some “usage notes”. Here’s what this looks like for the interoperability need of representing laboratory tests and observations:
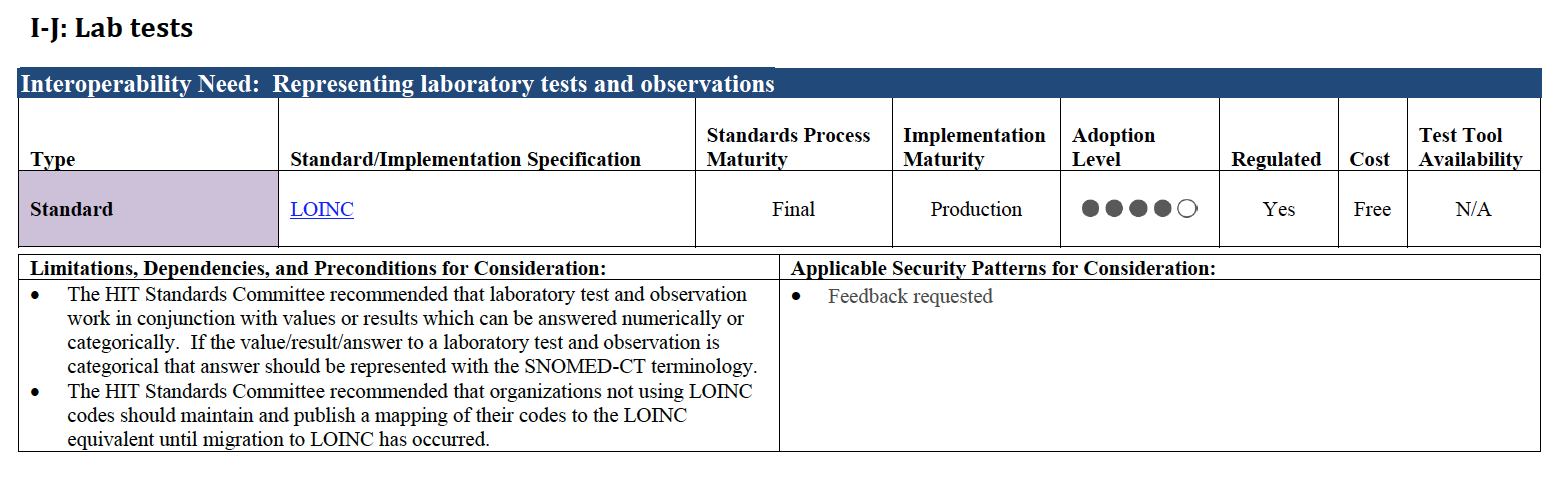
It is difficult to empirically determine adoption level for many standards (including LOINC). Nevertheless, including these attributes (even with rough approximations) helps make clear what ONC’s perception is and is intended to inform the dialogue about a standard’s “readiness” for use.
Questions and answers
From my perspective, the biggest flaw in the draft 2016 Advisory is that it does not recognize the prevailing two-part “observation” plus “observation value” information model for recording, storing, and transmitting lots of health data.
Specifically, the Advisory should make clear which vocabulary standard is needed for the observation, and which for categorical (coded) observation values.
The long-standing, well-established basic approach is that codes for observables should be drawn from LOINC, and codes for observation values should be drawn from other vocabularies, most often SNOMED CT. (There are a few cases, such as in genetic testing, where the result value is best communicated as an expression in a formal syntax such as HGVS or ISCN).
This two-part “question/answer” model is ubiquitous, was recommended across many domains by the HITSC, and is jointly endorsed by Regenstrief and the IHTSDO in their collaborative agreement.
In some content areas this pattern is the norm. Other times a single code, assertion style model is the norm, but the observation + observation value pattern is also seen.
The content areas in the 2016 Advisory where the observation + observation value pattern should be noted include:
- Care team member
- Race and ethnicity
- Family health history
- Functional status/disability
- Gender identity, sex, and sexual orientation
- Lab tests
- Smoking status
Content domains that should be added
The 2016 Advisory is missing several content domains that have clear interoperability needs and mature vocabulary standards. Specifically, I recommend the following new domains (interoperability needs) be added to the Advisory.
Clinical measurements and observations
Interoperability need: send, receive, and use clinical measurements and observations
Standard: LOINC
Interoperability need: send, receive, and use clinical measurement and observation result values
Standard: SNOMED CT
Clinical document types
Interoperability need: send, receive, and use clinical documents
Standard: LOINC
Patient reported outcome measures, survey instruments, and other standardized patient assessments
Interoperability need: send, receive, and use patient reported outcomes measures, survey instruments and patient assessments
Standard: LOINC

